Data Analytics
Our data scientists and database engineers will help you organize your quality-related event data (including errors, adverse events, near misses, unsafe conditions) and data from other specialized surveillance programs. We maximize the value of the information your organization has already collected.
federal adverse event reporting system Database requests
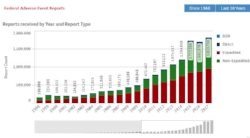
FAERS - We will help you search, organize and analyze information from the FDA's primary portal for adverse event information about medications. The FAERS database includes approximately 15 million reports, with more than 8 million serious reports and 1.5 million death reports.
Freedom of Information Requests (FOI)
FOI - We will help you write and submit FOI requests to federal and state agencies to access those difficult to find public facts about medication and medical device related information. Then, we help you organize and analyze the results.